The Global CHIVA Program is a groundbreaking medical education, training, and assessment platform for venous insufficiency diagnosis and treatment.
This program establishes the first globally recognized and certified standard for mastering the CHIVA technique, enabling healthcare practitioners worldwide to address venous diseases.
In collaboration and coordination with Dr. Smile Medical Group, Inteleos is set to launch the program in 2025.
CHIVA, first described by Professor Claude Franceschi in 1988, is a non-destructive alternative to common procedures for varicose veins. The aim of the technique is to lower transmural pressure in the superficial venous system and avoid destruction of veins while decreasing pain, cost, and nerve damage
CHIVA is the French acronym for “Cure conservatrice et Hemodynamique de l’Insuffisance Veineuse en Ambulatoire” (Conservative and Hemodynamic treatment of Venous Insufficiency in outpatients).
Sign up and be the first to receive program updates including program release dates.
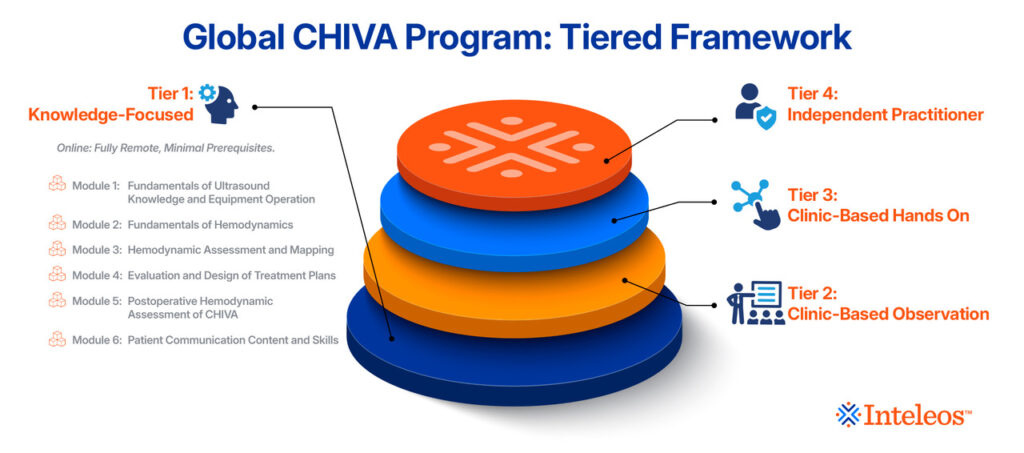
The goal of the Global CHIVA Program is to establish one cohesive framework to support CHIVA practitioners to address the health needs of patients with venous diseases on a global scale through a holistic and multidimensional, tiered approach.
Tier 1: Knowledge-Focused
Focus on delivering knowledge-based education and training content virtually through a customized Global CHIVA online learning management system (LMS), with the goal of building a foundational understanding of CHIVA and related medical knowledge.
The virtual online delivery platform includes e-learning modules, webinars, and other educational and training materials accessible to a wide audience around the globe.
Registered students and trainees will be awarded Tier 1 Certification only after successfully completing the required education and training content and then passing the assessment via the Global CHIVA Program LMS platform.
A Tier 1 Certificate is the prerequisite for entrance into the Tier 2 and 3 Programs.
Tier 2 Clinic-Based Observation
The Tier 2 Program will focus on observation and simulation exercises in the clinical setting. Training and education in qualified clinics will provide eligible healthcare professionals with practical exposure through on-site training sessions, simulation activities , and relevant workshops. This tier emphasizes learning by observing real-world scenarios and participating in structured simulation exercises within appropriate healthcare environments.
Tier 3: Clinic-Based Hands-on
The Tier 3 Program will concentrate on hands-on performance under supervision. Eligible participants will gain direct clinical experience by performing procedures and tasks in real-time settings, guided by experienced professionals. This immersive training will include practical workshops and supervised clinical practice, ensuring skill development through active participation in patient care.
Tier 4: Independent Practitioner
Designed to facilitate the transition of eligible and qualified participants into independent practitioners.
This will involve advanced on-site training, mentorship programs, and certification processes to help practitioners to perform autonomously comprehensive health interventions for patients in need.
Independent medical practitioners must meet all necessary legal requirements in their applicable countries and regions.
Tier 1: Knowledge-Focused
Tier 2: Clinic-Based Observation
Tier 3: Clinic-Based Hands On
Tier 4: Independent Practitioner
Tier 1: Knowledge-Focused
- Online: virtual/remote.
- Minimum prerequisites.
- Six (6) modules.
Module 1: Fundamentals of Ultrasound Knowledge and Equipment Operation
Module 2: Fundamentals of Hemodynamics
Module 3: Hemodynamic Assessment and Mapping
Module 4: Evaluation and Design of Treatment Plans
Module 5: Postoperative Hemodynamic Assessment of CHIVA
Module 6: Patient Communication Content and Skills
The Global CHIVA Advisory Panel consists of CHIVA subject matter experts from around the world who are contributing their expertise to support the development of the Global CHIVA Program.
The panel is used as a sounding board, and members may be asked to make recommendations or to provide important insights related to the CHIVA practices.
| Name | Country |
|---|---|
| Dr. Maria Caminati | Italy |
| Dr. Massimo Cappelli | Italy |
| Dr. Roberto Cuaranta | Spain |
| Dr. Michel Dadon | France |
| Dr. Jianping Deng | China |
| Dr. Luca De Siena | Italy |
| Dr. Roberto Delfrate | Italy |
| Dr. Xin Du | China |
| Dr. Stefano Ermini | Italy |
| Dr. José María Escribano | Spain |
| Dr. Felipe Puricelli Faccini | Brazil |
| Dr. Davide Foresti | Italy |
| Dr. Guglielmo Fornasari | Italy |
| Dr. Claude Franceschi | France |
| Dr. Gelfrido Galizi | Italy |
| Dr. Sergyi Kryzhanovskyi | Ukraine |
| Dr. Andres Jorge Kupelian | Argentina |
| Dr. Thanh-Phong Le | Vietnam |
| Dr. Erika Mendoza | Germany |
| Dr. Domenico Migaldi | Italy |
| Dr. Fausto Passariello | Italy |
| Dr. Mauro Pinelli | Italy |
| Dr. Juha Pitkaenen | Finland |
| Dr. Manuel Eugenio Senín Fernández | Spain |
| Dr. Qiang Zhang | China |
| Dr. Xiaoyin Zhu | China |
(If you are a member and would like your name removed from this list, please contact volunteer@inteleos.org)
Are you an expert in CHIVA who would like to become part of the Advisory Panel? Contact us at volunteer@inteleos.org.
2025
The 1st China Greater Bay Area Varicose Vein CHIVA Academic Salon
(January 4, 2025) Zhang Qiang Medical Group (Shenzhen) Varicose Vein CHIVA Center
2024
(December 2024), Beijing, China
The 25th Congress of the Asian Society for Vascular Surgery (ASVS)
(December 3-6, 2024) Bangkok, Thailand
American Vein & Lymphatic Society’s 38th Annual Congress
(October 10-14, 2024) Chicago, United States
AIIFEM – The 1st International Congress of the Italian and International Academy of Hemodynamic Phlebology
(October 18-19, 2024) Bari, Italy
Franceschi Academic Salon (FAS)
(April 20, 2024) Jinan, China
China-Africa Venous Webinar 2024
(July 27, 2024) Virtual
2023
(November 11, 2023)
2023 Clinical Practice Guidelines
Summary:
The 2023 clinical practice guidelines jointly developed by the Society for Vascular Surgery (SVS), American Venous Forum (AVF), and American Vein and Lymphatic Society (AVLS), and endorsed by the Society of Interventional Radiology (SIR) and the Society for Vascular Medicine (SVM), mark a significant milestone in the management of varicose veins of the lower extremities.
For the first time, the guidelines include the CHIVA technique in Section 6, which addresses interventions to preserve the great saphenous vein (GSV).
The guidelines state: “For patients with symptomatic varicose veins, we suggest preserving the GSV using the ambulatory conservative hemodynamic correction of venous insufficiency (CHIVA) technique, if performed by a physician who is familiar with the strategy.”
This inclusion highlights CHIVA as a minimally invasive, vein-conserving approach that underscores its growing acceptance and potential benefits in contemporary vascular care.
Learn more about the CHIVA approach to treating varicose veins directly from Dr. Smile.
This presentation by Dr. Smile, of the Dr. Smile Medical Group in China, gives an overview of how he has applied CHIVA technology to treat lower extremity varicose veins. A key part of this is the use of ultrasound hemodynamic assessments to develop treatment plans. This approach reduces venous pressure by recorrecting reflux and preserving an intact venous network.
The veins in the lower extremities are examined by the doctor using an ultrasound device in office. The inspection contents include whether the deep and superficial veins are patency, whether there is valvular regurgitation and the location of the escaping points and re-entry points.
Patients with varicose veins undergo outpatient treatment under local anesthesia. Dr. Smile Medical Group has established venous disease centers in 13 cities in China with the same standards and procedures, providing for safer and more efficient minimally invasive treatment for thousands of varicose vein patients every year.

